

National Qualifications - Sciences blog
OK Chemistry Teachers - here’s a little Christmas challenge to get you in the mood for teaching ‘open-ended questions’ in the new CfE-inspired Higher Chemistry!
All you have to do is to watch the video-clip above from a ‘CSI:Miami’ episode entitled ‘Dissolved’ (you’ll soon see why it got that name!) and then try to answer the open-ended question below! Remember that there are many correct answers, and the purpose is to get you to exercise your thinking skills! Have fun!
An internet discussion board on ‘Bad Chemistry’ has an entry referring to the TV drama ‘CSI: Miami’.
‘Last night’s episode showed the deceased victim floating in a swimming pool contaminated with sodium hydroxide. The concentration was high enough to eat through glass. When the CSI guys realised it was an alkali, they needed to neutralise it to retrieve the body, so they sent one of the team to the local grocery store for vinegar. They proceeded to pour the vinegar from four litre jugs into the pool, dropping the pH from 13 to exactly 7.0 – all within a few seconds, and without any stirring!’
Using your knowledge of chemistry, comment on whether the events described in CSI: Miami could take place.
More Open-ended questions in the new Higher Physics were the topic for a CPD session held at Marr College, South Ayrshire on the 14th December 2010. The session allowed physics teachers to consider the benefits and challenges offered by incorporating these questions into the curriculum.
Open-ended questions in the new Higher Physics were the topic for a CPD session held at Marr College, South Ayrshire on the 14th December 2010. The session allowed physics teachers to consider the benefits and challenges offered by incorporating these questions into the curriculum.
The ‘open-ended questions teacher’s guide’ housed on the NQ website was the basis of the core activities during this session. We’d love to hear your thoughts and comments!
More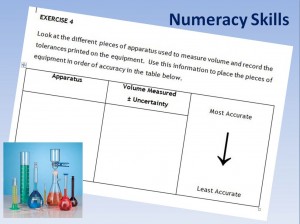 ’Building the Curriculum 4′ (the skills framework) states that every learner is entitled to the opportunity to develop skills for learning, life and work. This entitlement extends right through from the early years to the senior phase.
’Building the Curriculum 4′ (the skills framework) states that every learner is entitled to the opportunity to develop skills for learning, life and work. This entitlement extends right through from the early years to the senior phase.
As a result, one of the greatest challenges facing the Qualification Design Teams (QDTs) is to ensure that skills development is given a high priority within the new National 4/5 qualifications. Which skills are key within each subject, how will they be assessed, and how can teachers be supported in delivering these skills?
The process of skills development within the new CfE-inspired Higher Sciences was spotlighted at a recent meeting of QDT reps. It was noted that key ’generic’ skills were embedded throughout the course, but delivered within a specific scientific context. For example, numeracy skills were more particularly described as ‘skills of processing and analysing scientific data’; literacy skills were ’skills of writing an effective scientific communication’; communication skills included ‘carrying out effective-web-research’ or ‘producing an investigation report as a video/ scientific poster/ report/ etc’; higher-order thinking skills were promoted through the use of ‘open-ended questions’…
A number of the latest Higher Science resources supporting skills development were used to illustrate the types of support which might be provided by LTS for N4/5 qualifications. The Powerpoint Presentation used is available in the ‘presentations’ page on the National Qualifications Glow group under the heading ‘LTS/SQA Joint Event SKILLS’.
We’d love to hear your thoughts on how skills should be delivered in the new NQs!
More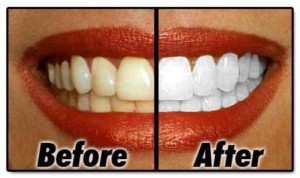 ‘Open-ended questions’ will feature in the new SQA Higher examinations in Chemistry and Physics (see earlier posts). These questions have no fixed response, and are designed to promote deeper understanding by encouraging learners to really think, rather than to merely recall facts.
‘Open-ended questions’ will feature in the new SQA Higher examinations in Chemistry and Physics (see earlier posts). These questions have no fixed response, and are designed to promote deeper understanding by encouraging learners to really think, rather than to merely recall facts.
Sixty recent Higher Chemistry students from six diverse Scottish secondaries participated in preliminary trials of these questions in June 2010. Their responses demonstrated impressive creativity and strong lateral thinking skills, as illustrated in the following selection of responses to a topical open-ended question on ‘teeth-whitening gels’:
Q/ Hydrogen peroxide is used in gels to whiten teeth. The ion-electron equation for the oxidation of hydrogen peroxide is shown below. Using your knowledge of chemistry, comment on possible methods for measuring and comparing the concentration of hydrogen peroxide present in two different gels.
![]()
Student 1:
’For a general idea of concentration, make a solution from the two different types of gel. Add potato disks, which contain the enzyme catalase, to catalyse the above reaction. Add washing -up liquid to both solutions, and the oxygen will form a foamy top layer. The gel solution with the higher volume of foam has the higher concentration of hydrogen peroxide.’
Student 2:
Student 3:
Hydrogen peroxide is a bleach, therefore the concentration could be determined by soaking a dyed material in equal volumes of the two gels for a fixed time. By finding out which one had the stronger bleaching effect, the concentrations could be compared.
Student 4:
Add Universal Indicator to both gels. The colour formed would show their pH. The lower the pH the more acidic, so the higher the concentration of hydrogen peroxide.
Student 6:
Stick a piece of the same tooth or bone material into the two gels for the same amount of time. The gel which has whitened the tooth or bone the most has the highest concentration of hydrogen peroxide in it.
What do you think? An astoundingly diverse array of excellent were produced when pupils were given the freedom to really think!
In a later post, we’ll look at the marking of open-ended questions.
MoreOpen-ended questions will be included in the new Physics and Chemistry Highers. They are designed to promote creative thinking and to challenge students to take the initiative and push the boundaries in their own learning.
This short, thought-provoking video from the TED Talks series shows American maths teacher Dan Meyer deconstructing traditional Physics and Maths questions, in order to generate discussion and engage learners through real life situations. His ideas are relevant for engaging learners in Biology and Chemistry at all levels, as well as in Physics.
One of the most interesting things Meyer does is to boil the maths/ physics question down to an everyday context, thereby opening it up to students’ own experience and engaging learners. In this way, Meyer states that every pupil can contribute to discussing and solving a maths/physics problem, whereas in the past they ‘switched off’.
Does this parallel what we are trying to achieve with open-ended questions?
More One of the main objectives of the current revision of the new Higher Sciences is to promote deeper understanding, creativity and analytical thinking, in line with the principles of Curriculum for Excellence. In order to help achieve this, SQA plan to include a small number of so-called ’open-ended questions’ within the new Higher examinations in Chemistry and Physics .
One of the main objectives of the current revision of the new Higher Sciences is to promote deeper understanding, creativity and analytical thinking, in line with the principles of Curriculum for Excellence. In order to help achieve this, SQA plan to include a small number of so-called ’open-ended questions’ within the new Higher examinations in Chemistry and Physics .
Two excellent resources entitled ‘Support Materials for Open Ended Questions in Higher Chemistry‘ and ‘Support Materials for Open Ended Questions in Higher Physics’ have recently been published on the NQ Support page of the LTS website. These resources provide a rationale for the introduction of OEQs in each subject, describe what ‘open-ended questions’ are, give a number of examples of questions with sample pupil answers, and advise on the marking of open-ended questions.
More information from recent extensive pupil trials of open-ended questions to follow in another post!
More
Find us on