

National Qualifications - Sciences blog
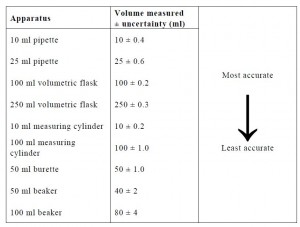 Chemistry Teachers are spoiled for choice with fabulous new resources these days! Check out yet another brand-new resource supporting skills development within the ‘Researching Chemistry’ unit of the revised Higher Chemistry.
Chemistry Teachers are spoiled for choice with fabulous new resources these days! Check out yet another brand-new resource supporting skills development within the ‘Researching Chemistry’ unit of the revised Higher Chemistry.
The resource consists of a pupil activity pack and a teacher’ guide. It enables students to develop and practice the key skills of ‘Processing and Analysing Data’ which are essential to effectively carrying out an investigative task at Higher Chemistry level. The resource addresses a range of important topics such as plotting scatter graphs, the benefits of carrying out replicate measurements, identification and elimination of ‘rogue points’, calculating mean values, appreciating the relative accuracy of volumetric apparatus, qualitative understanding of uncertainty, etc.
This resource would also provide an excellent preparation for current Advanced Higher students in the skills required to successfully write-up their investigation report.
Let us know if you like it!
More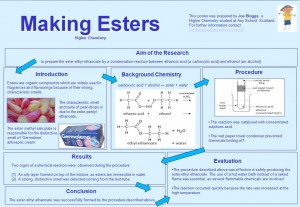 Calling all Chemistry Teachers! Check out a brilliant new skills resource to support pupils undertaking the ‘Researching Chemistry’ unit of the new Higher. The purpose of this resource is to enable students to develop the skills of effective scientific communication within a chemistry context.
Calling all Chemistry Teachers! Check out a brilliant new skills resource to support pupils undertaking the ‘Researching Chemistry’ unit of the new Higher. The purpose of this resource is to enable students to develop the skills of effective scientific communication within a chemistry context.
The resource provides opportunities to learn and practice the key skills of scientific writing (e.g. writing in the past tense/passive voice), to identify the key stages in all forms of scientific communication (aim, procedure, results, etc), and to evaluate the effectiveness and appropriateness of a number of traditional (e.g. lab-report, scientific paper, PowerPoint presentation, scientific poster) and more modern means of scientific communication (videos, blogs, podcasts, etc).
This resource will help prepare Higher Chemistry students to independently produce their own effective scientific communication at the end of the ‘Researching Chemistry’ half-unit. The resource would also be very helpful for current Advanced Higher students preparing to write up their investigation unit.
Let us know what you think about this resource!
More Science Development Officers representing Biology, Chemistry and Physics have been very busy this week delivering CPD to hundreds of science teachers from Aberdeenshire and Highland regions on their subject INSET days.
Science Development Officers representing Biology, Chemistry and Physics have been very busy this week delivering CPD to hundreds of science teachers from Aberdeenshire and Highland regions on their subject INSET days.
The venue for the Aberdeenshire teachers’ meeting was Westhill Academy and the Highland teachers’ meeting was held in Millburn Academy in Inverness.
The main focus of the CPD was an update on the latest progress in the revised Highers in the four sciences. The powerpoint presentations from these events will be made available in the NQ Science Glow Groups by the end of this week.
More Good news for teachers of Higher Chemistry and Higher Physics! Free, printed versions of three brand-new resources for each subject have been distributed to all Scottish schools this week! Look out for these in a mailbox near you soon!
Good news for teachers of Higher Chemistry and Higher Physics! Free, printed versions of three brand-new resources for each subject have been distributed to all Scottish schools this week! Look out for these in a mailbox near you soon!
The online version of these resources are also available on the National Qualifications site on the LTS website. These are just a sample of the many excellent new resources which have been produced to support the new Highers in Physics and Chemistry.
The titles which have been distributed in print for Higher Chemistry are;
For Higher Physics, the printed resources which have been sent to schools are;
Let us know if you find these useful!
MoreOK Chemistry Teachers - here’s a little Christmas challenge to get you in the mood for teaching ‘open-ended questions’ in the new CfE-inspired Higher Chemistry!
All you have to do is to watch the video-clip above from a ‘CSI:Miami’ episode entitled ‘Dissolved’ (you’ll soon see why it got that name!) and then try to answer the open-ended question below! Remember that there are many correct answers, and the purpose is to get you to exercise your thinking skills! Have fun!
An internet discussion board on ‘Bad Chemistry’ has an entry referring to the TV drama ‘CSI: Miami’.
‘Last night’s episode showed the deceased victim floating in a swimming pool contaminated with sodium hydroxide. The concentration was high enough to eat through glass. When the CSI guys realised it was an alkali, they needed to neutralise it to retrieve the body, so they sent one of the team to the local grocery store for vinegar. They proceeded to pour the vinegar from four litre jugs into the pool, dropping the pH from 13 to exactly 7.0 – all within a few seconds, and without any stirring!’
Using your knowledge of chemistry, comment on whether the events described in CSI: Miami could take place.
MoreCheck out an exciting new set of resources to support the ‘Consumer Chemistry’ and ‘Periodicity, Polarity & Properties’ units of the new CfE-inspired Higher Chemistry. The resource consists of an interactive PowerPoint presentation which could be used to introduce the concepts involved, plus a sample set of pupil problems, with associated answers.
The resources support learners and teachers with a new style of exam question which the SQA will include in the CfE-inspired Higher Chemistry. This new question style requires students to identify key functional groups within complex organic molecules, and then to link the presence of certain functional groups to the molecule’s physical properties. Physical properties such as solubility, melting and boiling point and viscosity are included.
The resources can be found both on the National Qualifications website and on the NQ Chemistry Glow Group, and could be used to support aspects of the current and the CFE-inspired Highers in Chemistry.
Let us know if you find them useful!
More The new CfE-inspired Higher Sciences specifically develop a number of crucial transferable skills for life and work. In the Physics and Chemistry Highers these skills are gained through the ‘Researching’ half-units, whereas in the Biology and Human Biology Highers they are developed through the ‘case studies’. A mixture of scientific and generic skills are developed throughout the new courses.
The new CfE-inspired Higher Sciences specifically develop a number of crucial transferable skills for life and work. In the Physics and Chemistry Highers these skills are gained through the ‘Researching’ half-units, whereas in the Biology and Human Biology Highers they are developed through the ‘case studies’. A mixture of scientific and generic skills are developed throughout the new courses.
One key skill for any scientist is to be able to undertake an effective literature search to find out what is already known about their topic of interest. Web-based research is becoming an increasingly important tool for scientists. Two superb resources (an introductory powerpoint and a set of pupil activities) have been prepared to support teachers and learners with the development of effective web-based research skills in chemistry. These resources can also be found on the NQ Chemistry Glow group.
Watch this space for news on other brand-new resources for the development of other key scientific skills….!
More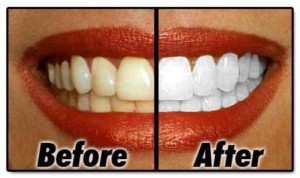 ‘Open-ended questions’ will feature in the new SQA Higher examinations in Chemistry and Physics (see earlier posts). These questions have no fixed response, and are designed to promote deeper understanding by encouraging learners to really think, rather than to merely recall facts.
‘Open-ended questions’ will feature in the new SQA Higher examinations in Chemistry and Physics (see earlier posts). These questions have no fixed response, and are designed to promote deeper understanding by encouraging learners to really think, rather than to merely recall facts.
Sixty recent Higher Chemistry students from six diverse Scottish secondaries participated in preliminary trials of these questions in June 2010. Their responses demonstrated impressive creativity and strong lateral thinking skills, as illustrated in the following selection of responses to a topical open-ended question on ‘teeth-whitening gels’:
Q/ Hydrogen peroxide is used in gels to whiten teeth. The ion-electron equation for the oxidation of hydrogen peroxide is shown below. Using your knowledge of chemistry, comment on possible methods for measuring and comparing the concentration of hydrogen peroxide present in two different gels.
![]()
Student 1:
’For a general idea of concentration, make a solution from the two different types of gel. Add potato disks, which contain the enzyme catalase, to catalyse the above reaction. Add washing -up liquid to both solutions, and the oxygen will form a foamy top layer. The gel solution with the higher volume of foam has the higher concentration of hydrogen peroxide.’
Student 2:
Student 3:
Hydrogen peroxide is a bleach, therefore the concentration could be determined by soaking a dyed material in equal volumes of the two gels for a fixed time. By finding out which one had the stronger bleaching effect, the concentrations could be compared.
Student 4:
Add Universal Indicator to both gels. The colour formed would show their pH. The lower the pH the more acidic, so the higher the concentration of hydrogen peroxide.
Student 6:
Stick a piece of the same tooth or bone material into the two gels for the same amount of time. The gel which has whitened the tooth or bone the most has the highest concentration of hydrogen peroxide in it.
What do you think? An astoundingly diverse array of excellent were produced when pupils were given the freedom to really think!
In a later post, we’ll look at the marking of open-ended questions.
MoreThe ‘Periodicity, Polarity & Properties’ half-unit of the revised Higher Chemistry contains many ‘traditional’ areas of chemistry which will be familiar to teachers of the current Higher e.g. structure & bonding, electronegativity, bond polarity, intermolecular forces, patterns in the periodic table, etc.
These key topics are included in the modernised Higher because they are fundamentally important to the understanding of all other areas of chemistry. However, pupils can often struggle to deal with the abstract concepts involved, and can sometimes find these topics dry.
A brand-new set of animated powerpoint resources to enliven the learning & teaching of some of these more theoretical topics has just been published today on the National Qualifications page on the LTS website. These attractive and engaging resources are suitable for use with both the current and the revised chemistry Highers, and cover the following topics:
Watch this space for animations on intermolecular forces later in the year!
The resources could be used to introduce these topics, or as a revision resource for learners. Enjoy!
Moreeight="364">
The new Highers in Chemistry and Physics contain a ‘Researching Chemistry’ half-unit in which students learn about the key skills of scientific enquiry, and then apply and develop these skills via investigation into a topical scientific issue.
Teachers will find the BBC video clip above from the TV programme ‘Bang Goes the Theory’ on ‘How to Investigate the Unknown’ to be a useful introduction to the concepts involved in scientific research. It is based on research into factors affecting the effectiveness of ‘canister rockets’, but could be re-applied to any other research topic.
The weblink also gives interesting additional background reading and suggested activities. The resource could also be used to introduce investigative activities in any scientific context, especially Adavanced Higher investigations.
Enjoy!
More
Find us on